Two recent clinical trials studying Invokana (canagliflozin) shed light on the diabetes drug’s alarming side effects. Canagliflozin is a type 2 sodium-glucose transport inhibitor (SGLT2 inhibitor) marketed by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson. The medication works to lower blood sugar levels in the body by stopping the kidneys from reabsorbing blood glucose. Instead of the blood glucose staying in the body, it is removed with the body’s urine.
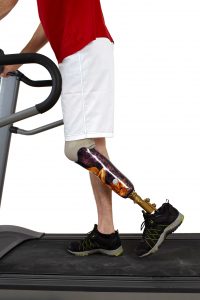
The clinical trials were named CANVAS and CANVAS-R, based on long scientific acronyms. The studies examined the effects of canagliflozin on patients with Type-2 diabetes. The trials discovered that leg and foot amputations occurred twice as often in patients taking canagliflozin as those treated with a placebo.
The risk for amputations broke down like this: 5.9 out of every 1,000 patients treated with canagliflozin suffered amputation, as compared to 2.8 out of every 1,000 patients treated with a placebo. Over a year’s time, the risk of amputation was 7.5 out of every 1,000 patients treated with canagliflozin, compared to 4.2 out of every 1,000 patients treated with a placebo. These are statistically significant results, meaning the risk of amputation for those people taking Invokana was large enough to cause alarm in the medical community.
In the clinical trials, amputations of the toe and middle of the foot were the most common; however, amputations of the leg, below and above the knee, also occurred. Some patients had more than one amputation.
Based on this new data, the FDA ordered new warnings, including a prominent boxed warning, to be added to the canagliflozin drug labels to explain and describe this risk. Continue reading
 Invokana is a drug prescribed to treat people with Type 2 diabetes. The medication lowers blood sugar levels by preventing the kidneys from reabsorbing blood glucose. I’ve written often about Invokana and the studies that have identified problems with the drug, which you can read about here. I thought it may be useful to give you a history of key dates in the life-cycle of the drug, from its market release through the latest developments in the multidistrict litigation, where currently 1,000 lawsuits have been filed.
Invokana is a drug prescribed to treat people with Type 2 diabetes. The medication lowers blood sugar levels by preventing the kidneys from reabsorbing blood glucose. I’ve written often about Invokana and the studies that have identified problems with the drug, which you can read about here. I thought it may be useful to give you a history of key dates in the life-cycle of the drug, from its market release through the latest developments in the multidistrict litigation, where currently 1,000 lawsuits have been filed. North Carolina Product Liability Lawyer Blog
North Carolina Product Liability Lawyer Blog