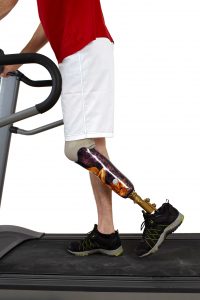
Two recent clinical trials studying Invokana (canagliflozin) shed light on the diabetes drug’s alarming side effects. Canagliflozin is a type 2 sodium-glucose transport inhibitor (SGLT2 inhibitor) marketed by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson. The medication works to lower blood sugar levels in the body by stopping the kidneys from reabsorbing blood glucose. Instead of the blood glucose staying in the body, it is removed with the body’s urine.
The clinical trials were named CANVAS and CANVAS-R, based on long scientific acronyms. The studies examined the effects of canagliflozin on patients with Type-2 diabetes. The trials discovered that leg and foot amputations occurred twice as often in patients taking canagliflozin as those treated with a placebo.
The risk for amputations broke down like this: 5.9 out of every 1,000 patients treated with canagliflozin suffered amputation, as compared to 2.8 out of every 1,000 patients treated with a placebo. Over a year’s time, the risk of amputation was 7.5 out of every 1,000 patients treated with canagliflozin, compared to 4.2 out of every 1,000 patients treated with a placebo. These are statistically significant results, meaning the risk of amputation for those people taking Invokana was large enough to cause alarm in the medical community.
In the clinical trials, amputations of the toe and middle of the foot were the most common; however, amputations of the leg, below and above the knee, also occurred. Some patients had more than one amputation.
Based on this new data, the FDA ordered new warnings, including a prominent boxed warning, to be added to the canagliflozin drug labels to explain and describe this risk.
Black Box Warnings
A boxed warning is serious business. A “boxed warning” or “black box warning” is a warning that appears on the package insert for certain prescription drugs. It is given this name because the FDA requires that the warning be presented with a box or notable border around the text. The boxed warning is designed to call attention to serious or life-threatening risks that are possible when using the prescription drug.
In July 2017, the FDA required a boxed warning for the diabetes drug Invokana, which included these bullet points:
“Warning: Lower Limb Amputation”
- A 2-fold increased risk of lower limb amputations was observed in two studies of patients taking Invokana.
- Amputations of the toe and midfoot were most frequent; amputations of the leg were also observed. Some patients had multiple amputations.
- Before taking Invokana, consider factors that may increase the risk of amputation, such as a history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers.
- Monitor patients receiving Invokana for infection, new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue if these complications
occur.
See FDA Website for the complete boxed warning.
The bottom line: if you are taking Invokana for treatment of Type-2 diabetes, talk to your doctor about the risks and whether you should remain on the medication. For more information, consult the FDA website.
 North Carolina Product Liability Lawyer Blog
North Carolina Product Liability Lawyer Blog