Artificial hips offer many patients the opportunity to live active and full lives, but sometimes they don’t always work as intended. Thankfully, these are relatively rare situations. Unfortunately, if it’s your artificial hip that fails, there’s not much comfort in knowing the artificial hips in most other patients work as designed. Because an artificial hip failure can be such a serious problem, the U.S. Food and Drug Administration (FDA) takes steps to ensure artificial hip medical devices receive adequate review and scrutiny before being approved for use in patients. One such step is to confirm that if a company modifies an already approved product, those changes also receive appropriate FDA approval and oversight. Apparently, Synovo Production, Inc. (Synovo) failed to obtain these necessary FDA approvals after making changes to one of Synovo’s hip replacement products. Let’s take a look at what products are affected and what action the FDA has taken.
Artificial hips offer many patients the opportunity to live active and full lives, but sometimes they don’t always work as intended. Thankfully, these are relatively rare situations. Unfortunately, if it’s your artificial hip that fails, there’s not much comfort in knowing the artificial hips in most other patients work as designed. Because an artificial hip failure can be such a serious problem, the U.S. Food and Drug Administration (FDA) takes steps to ensure artificial hip medical devices receive adequate review and scrutiny before being approved for use in patients. One such step is to confirm that if a company modifies an already approved product, those changes also receive appropriate FDA approval and oversight. Apparently, Synovo Production, Inc. (Synovo) failed to obtain these necessary FDA approvals after making changes to one of Synovo’s hip replacement products. Let’s take a look at what products are affected and what action the FDA has taken.
Affected Medical Devices
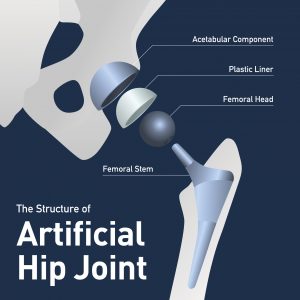
Synovo makes the Total Hip System (also known as the Total Hip Replacement System and the Preserve and Endotec BP), which the FDA cleared for medical use. However, the FDA claims that Synovo has made significant modifications to the following three components of this system: Femoral Resurfacing Cup, Acetabular Fixation Cup and Acetabular Bearing. Some of these components are also stand-alone products.
 North Carolina Product Liability Lawyer Blog
North Carolina Product Liability Lawyer Blog