 The Zimmer NexGen artificial knee has been causing patients much pain and suffering over the past decade. Zimmer first instituted a recall of one group of NexGen components in 2010, and there have been other recalls in the years following. Many patients with the NexGen knees have suffered pain and an inability to maintain normal physical activity. The lawsuits of course followed.
The Zimmer NexGen artificial knee has been causing patients much pain and suffering over the past decade. Zimmer first instituted a recall of one group of NexGen components in 2010, and there have been other recalls in the years following. Many patients with the NexGen knees have suffered pain and an inability to maintain normal physical activity. The lawsuits of course followed.
In August 2011, to handle the growing number of lawsuits over the NexGen knee, a multidistrict litigation (MDL) site was formed in Chicago, Illinois and assigned to federal district court judge Rebecca Pallmeyer. The MDL is titled: In Re: Zimmer NexGen Knee Implant Products Liability Litigation, No. 2272 (1:11-cv-5468).
Do You Qualify for the Zimmer NexGen MDL?
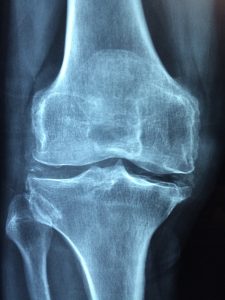
The overarching requirement to participate in the MDL is (1) to prove implantation of the Zimmer NexGen knee components (with product stickers from the original surgery), and (2) to show through the medical records clear evidence of loosening. Loosening is when the artificial knee components begin to move in the patient’s leg and separate from the bone. This is not good. Loosening can be very painful. A loose artificial knee will cause major complications and eventually require replacement and revision surgery. When a patient complains to an orthopedic surgeon about knee pain after a total knee arthroplasty, the doctor will order an X-ray. The doctor will look for radiolucent lines, which are spaces between the artificial knee component and the patient’s bone. Essentially, small gaps occur because the medical device is not implanted tightly or securely to the bone. These spaces can cause serious problems for a patient with an artificial knee, and can be the first signs of an artificial knee failure. The spaces often fill with fluid or tissue which can cause additional loosening of the medical device.
Zimmer NexGen Knee Was Approved Through the 510(K) Process
The Zimmer NexGen artificial knee bypassed the normal premarket testing for new medical products through a process known as “510(k).” I have written about this “rush to market” procedure on this site. The 510(k) process allows a manufacturer to notify the Food and Drug Administration under section 510(k) of the Medical Device Amendments Act of 1976 (MDA) of its intent to market a device (like the NexGen knee); the manufacturer must describe the new device’s “substantial equivalence” to a pre-MDA device. The FDA may then approve the new device for sale in the United States without rigorous testing as with new medical devices. This is how the Zimmer NexGen Knee reached the market.
Large numbers of people have suffered from pain and required revision surgery to replace the Zimmer NexGen artificial knees. Despite all this suffering, the MDL has moved very slowly. Bellwether cases have been selected, and trial dates assigned, only to be continued or otherwise postponed. For individuals who filed suit against Zimmer years ago (including some of my clients), it has been a frustrating exercise. Over the years, the qualifications in the MDL have narrowed, and the requirements to qualify for potential compensation have gotten more difficult. As a result, many lawsuits have been dismissed from the litigation as not qualifying under the latest criteria.
Case Management Order No. 11 (The “Lone Pine” Order)
On June 24, 2016, MDL Judge Rebecca Pallmeyer issued the latest case management order (CMO) which set out the latest requirements to determine if a case has “sufficient merit to proceed to trial.” The new order, which is referred to as a Lone Pine Order, requires each plaintiff to file an Expert Declarations form establishing that the case meets all the latest requirements to warrant its day in court (that is, a trial by jury). These requirements are complex and come from different court orders over several years, but I will try to set them out (in simplified form) here. The plaintiff must:
- File a Plaintiff Fact Sheet (which details the key facts of the case);
- Show Evidence of Loosening of the Zimmer NexGen knee;
- Submit Evidence of Knee Flexion of 120 Degrees or Greater;
- Identify the Case as Track One or Track Two (Track Two is essentially a case where the requirements of Track One have not been met);
- Designate Whether the Plaintiff Intends to Pursue Certain Specific Claims, Such as:
- Femoral loosening resulting from high flexion activities;
- Loosening of tibial component;
- Tibial loosening of MIS tibial component with a drop down stem.
I know, I know, what the heck does all this mean? It is highly technical, and an orthopedic surgeon or a competent medical device attorney is often necessary to make sense of these directives. For now, let me say that this case management order presents a new challenge for the plaintiffs involved in the litigation. These requirements are precise and rigorous and designed to weed out cases which do not have medical records clearly showing evidence of artificial knee loosening, along with injury and pain for the patient. Undoubtedly, this Lone Pine Order will cause some of the cases to be abandoned. But if you have a Zimmer NexGen knee implanted, and it failed, and the failure led to revision surgery, you should find a good product liability attorney to review your case thoroughly and see if it qualifies under the latest guidelines. But do not delay.
The first bellwether trial in the Zimmer NexGen MDL is scheduled for October 2016. Let’s hope it does not get postponed (again).
 North Carolina Product Liability Lawyer Blog
North Carolina Product Liability Lawyer Blog


