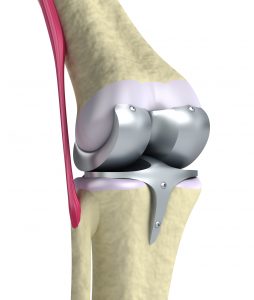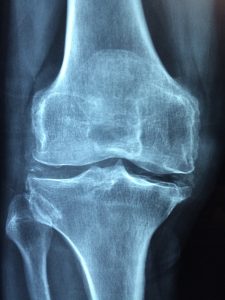
Without question, the Zimmer NexGen Knee MDL is not going all that well for plaintiffs lately. The first bellwether trial ended in a defense verdict in favor of Zimmer. Then Judge Rebecca Pallmeyer issued a Lone Pine Order which imposed a heavier burden on plaintiffs to avoid dismissal of their cases. That Order requires each plaintiff to file an Expert Declarations form establishing that the case meets all the latest requirements to warrant its continued place in the litigation. These requirements include a showing of (1) evidence of loosening of the artificial knee, (2) evidence of knee flexion of 120 degrees, (3) other detailed designations of injury and product failure. You can read more about the Lone Pine Order here.
Then, on October 21, 2016, Judge Pallmeyer ruled that the second bellwether case did not warrant a jury trial. In her Order, she granted summary judgment for Zimmer. You can read about that court decision here, but in a nutshell, Judge Pallmeyer simply rejected the validity of the plaintiff’s key expert witness. She concluded that Dr. Joseph Fetto failed to refer to scientific literature or to “give any explanation for why the implant design, and asymmetric loading generally, causes . . . loosening.” (Order, p. 12) She wrote at length about the reasons why Dr. Fetto’s testimony is unreliable, ultimately concluding that Dr. Fetto has not “given the court sufficient basis to conclude that his opinion is reliable.” (Order, p. 17) Without a reliable expert witness, a plaintiff cannot win a product liability case.
The Judge’s Order was a sledgehammer, but . . .
It’s Not All Bad News
It’s quite awful to select a bellwether case, prepare it for trial, and then, days before jury selection, the judge grants summary judgment on all claims in favor of defendants. After years of litigation, the plaintiff, who was clearly injured, was out of court without compensation. Still, there is reason to believe that future cases may have different results. Judge Pallmeyer admitted as much in the last section of her 43-page order, titled “Potential Differences Between Joas’s Case and Others in the MDL.” Let’s examine a few keys statements in the part of her Order (the italicized statements below were written by Judge Pallmeyer):
Continue reading
 Artificial hips offer a new lease on life to many thousands of people each year. They provide recipients the opportunity for independent living and mobility they might not otherwise have. Unfortunately, they also come with risks, one of the most prominent being metallosis. But there’s also another potential risk where the femur (thigh bone) could break.
Artificial hips offer a new lease on life to many thousands of people each year. They provide recipients the opportunity for independent living and mobility they might not otherwise have. Unfortunately, they also come with risks, one of the most prominent being metallosis. But there’s also another potential risk where the femur (thigh bone) could break. North Carolina Product Liability Lawyer Blog
North Carolina Product Liability Lawyer Blog