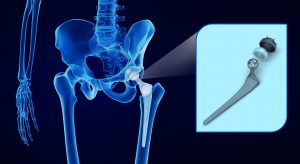
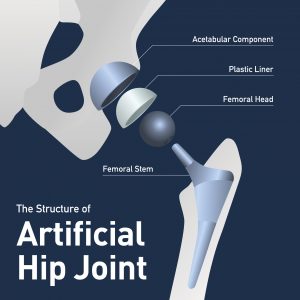
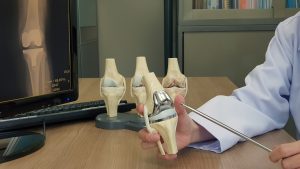
 Exactech Inc. (Exactech) is a company that makes various products to repair or replace joints in the human body. Some of Exactech’s biggest selling products have been replacement joints for hips, knees and ankles. Unfortunately, there have been some problems with certain products suffering from premature wear and other issues. This has led to some patients getting revision surgery to fix the issue and repair damage caused to the human body. Exactech has also started various product recalls involving certain knee, ankle and hip implants. I have prepared the following Exactech FAQs so you can learn more about this problem and what to do if you’re affected.
Exactech Inc. (Exactech) is a company that makes various products to repair or replace joints in the human body. Some of Exactech’s biggest selling products have been replacement joints for hips, knees and ankles. Unfortunately, there have been some problems with certain products suffering from premature wear and other issues. This has led to some patients getting revision surgery to fix the issue and repair damage caused to the human body. Exactech has also started various product recalls involving certain knee, ankle and hip implants. I have prepared the following Exactech FAQs so you can learn more about this problem and what to do if you’re affected.
1. What Exactech Products Have Been Recalled?
The recall involves two groups of products. The first group relates to certain batches of Exactech’s Connexion GXL acetabular hip liners. The second group relates to specific ankle and knee polyethylene liners and inserts, many of which were manufactured in 2004 or later. Some of these products have been sold under the following brands:
 North Carolina Product Liability Lawyer Blog
North Carolina Product Liability Lawyer Blog