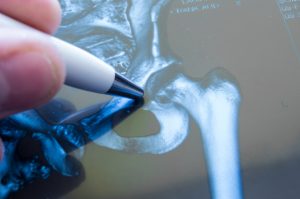
In the last three DePuy Pinnacle artificial hip bellwether trials, three juries awarded the following amounts of money: $502,000,000.00, $1,041,311,648.17, and $247,000,000.00. That’s a total of $1.79 billion dollars. The juries awarded plaintiffs compensatory damages (or actual damages) and punitive damages (to “punish” the defendant companies). Remember that these juries settled on these huge amounts of money based on their findings in three separate trials that DePuy and Johnson & Johnson were liable for design and manufacturing defects, that the defendants failed to warn plaintiffs about the risks of the defective artificial hip, and that defendants acted recklessly, intentionally, and even maliciously in marketing and selling the flawed DePuy Pinnacle hip. These last findings permitted the juries to award punitive damages.
In the last three DePuy Pinnacle artificial hip bellwether trials, three juries awarded the following amounts of money: $502,000,000.00, $1,041,311,648.17, and $247,000,000.00. That’s a total of $1.79 billion dollars. The juries awarded plaintiffs compensatory damages (or actual damages) and punitive damages (to “punish” the defendant companies). Remember that these juries settled on these huge amounts of money based on their findings in three separate trials that DePuy and Johnson & Johnson were liable for design and manufacturing defects, that the defendants failed to warn plaintiffs about the risks of the defective artificial hip, and that defendants acted recklessly, intentionally, and even maliciously in marketing and selling the flawed DePuy Pinnacle hip. These last findings permitted the juries to award punitive damages.
In the bellwether trial in March 2016, a jury awarded more than $500,000,000.00 to five plaintiffs. On December 1, 2016 a jury awarded more than one billion dollars to six plaintiffs and four spouses. And finally, just two weeks ago, a jury awarded six plaintiffs (and four spouses) $247,000,000.00 in compensatory and punitive damages. Compared to the total awards, the amounts awarded to the spouses of the hip victims were modest, and appear to have totaled around $6,700,000.00.
Let’s do a little math:
 After news broke that Zantac (the brand name for ranitidine) was linked to cancer, a large wave of lawsuits started making their way into state and federal courts. Many of these cases have been consolidated into the Zantac multi-district litigation, or MDL 2924.
After news broke that Zantac (the brand name for ranitidine) was linked to cancer, a large wave of lawsuits started making their way into state and federal courts. Many of these cases have been consolidated into the Zantac multi-district litigation, or MDL 2924. North Carolina Product Liability Lawyer Blog
North Carolina Product Liability Lawyer Blog